About the Studies
ReNEW Is Evaluating a New Investigational Treatment for Dry AMD
The ReNEW study is a global research study evaluating a new investigational treatment for dry AMD. The study will evaluate the efficacy of once-daily subcutaneous (SC) injections of the investigational therapy, elamipretide, in subjects who have dry age-related macular degeneration (dry AMD). The investigational medicine, elamipretide, will be compared to placebo.
The duration of the research study for the new investigational treatment for dry AMD will consist of a screening period, a masked treatment period of 96 weeks, and a safety follow-up period with the option for participants to enroll in the ReTAIN open-label extension (OLE) trial.

About the Investigational Medicine
The research study will evaluate a potential new treatment for dry AMD using the investigational therapy, elamipretide. Elamipretide is an investigational compound that readily penetrates cell membranes and targets the inner mitochondrial membrane, where it binds reversibly to cardiolipin. Elamipretide has been shown in preclinical studies to normalize mitochondrial structure and function and improve cell viability and organ function across a spectrum of disease models. These studies include models of cardiovascular, renal, metabolic, skeletal muscle, neurodegenerative, and genetic mitochondrial disease. Elamipretide is administered via subcutaneous injection.
The investigational medicine was previously assessed in a phase 2, randomized, double-masked, placebo-controlled clinical study (ReCLAIM-2) to evaluate the safety, efficacy, and pharmacokinetics of subcutaneous injections of elamipretide in subjects with dry AMD with geographic atrophy (GA), published in Ophthalmology Science (https://www.ophthalmologyscience.org/article/S2666-9145(24)00164-7/fulltext). While the trial did not meet its primary efficacy endpoints (mean change in GA area or low luminance visual acuity [LLVA]), elamipretide treatment was associated with,
- slowing of progressive ellipsoid zone (EZ) thinning & loss, an accepted surrogate for photoreceptor damage & loss and
- more patients receiving elamipretide experienced ≥2-line (≥10 letter) and ≥3-line (≥15 letter) gains in LLVA relative to placebo
Reference
Ehlers JP, Hu A, Boyer D, et al. ReCLAIM-2: A Randomized Phase II Clinical Trial Evaluating Elamipretide in Age-related Macular Degeneration, Geographic Atrophy Growth, Visual Function, and Ellipsoid Zone Preservation. Ophthalmol Sci. 2024;5(1):100628.
Investigational Dry AMD Treatment Study Participation
The duration of the research study will consist of a Screening Period, a Masked Treatment Period, and a Safety Follow-Up Period, with the option for participants to enroll in the ReTAIN open-label extension (OLE) trial.
Participation in the study consists of the following periods:
Screening Period
The study team will perform several assessments and procedures to determine whether interested individuals are eligible to participate.
Masked Treatment Period
This period lasts approximately 96 weeks. After randomization, participants will receive either study treatment or placebo treatment and attend visits at the study site for routine assessments and procedures. “Masked” means that neither you nor the study doctor will know whether you are receiving the investigational medication or the placebo.
Follow-Up Period
For subjects not enrolling in the ReTAIN open-label extension, the 4-week Safety Follow-Up Period will begin after the masked treatment period is completed. Subjects will return to the clinical site on Week 100 for a final safety assessment.

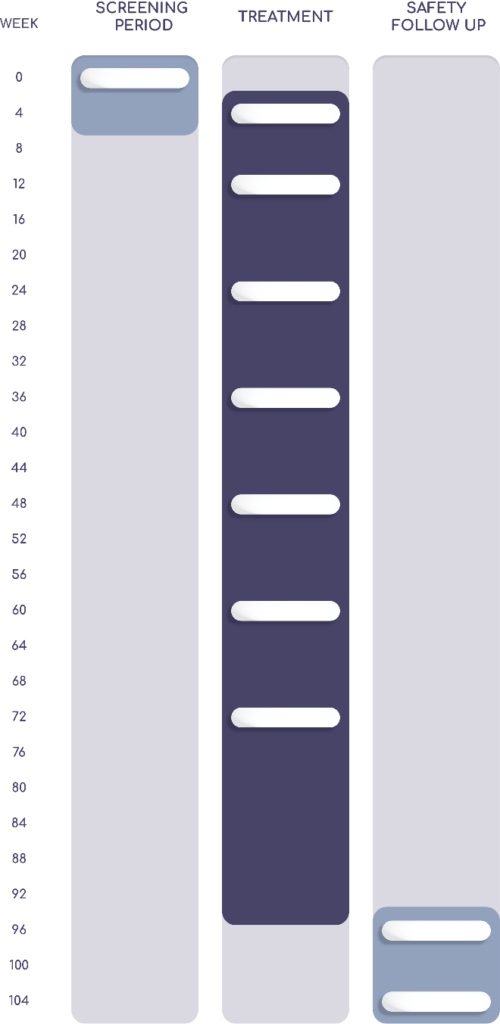
During the Treatment Period, study participants will return to the clinical site at Week 4, Week 12, Week 24, Week 36, Week 48, Week 60, Week 72, Week 84, and Week 96 for assessments. On non-visit days, subjects (or trained caregivers) will administer the investigational medicinal product or placebo daily during the Treatment Period and record it in a diary provided by the study sponsor. During the Treatment Period, subjects will continue to follow all trial requirements, including documenting the location and time of the daily injection in the diary.
